中国病毒学论坛|我们一直在坚持!
标题: Cell揭秘:免疫细胞得“吃点东西”,才能工作 [打印本页]
作者: ipsvirus 时间: 2016-6-13 16:50
标题: Cell揭秘:免疫细胞得“吃点东西”,才能工作
近日,Sciencedaily网站发表了题为“You are what you eat: Immune cells remember their first meal”的文章。报道指出,英国布里斯托大学的科学家们已经鉴定出了免疫细胞炎症反应的“触发器”,这一发现有望为开发针对多种人类疾病的新疗法铺平道路。相关研究于5月19日发表在Cell杂志上。
免疫细胞对机体的维持和修复至关重要。然而,尽管免疫响应有益于人类健康,但过度的免疫响应会造成包括动脉粥样硬化、癌症和关节炎等在内的多种疾病,同时还会加剧疾病的发展。因此,更多的了解免疫响应的激活机制对设计治疗这些炎症性疾病的新疗法非常关键。
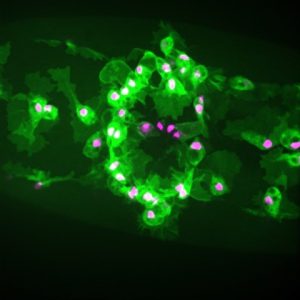
响应创伤的果蝇巨噬细胞
该研究的第一作者Helen Weavers博士说:“我们的研究发现,免疫细胞在能够响应创伤或感染前首先必须通过‘吃’一个垂死的邻近细胞来激活自己。这样一来,免疫细胞会建立关于‘这顿饭’的分子记忆,从而形成它们的炎症性行为。”
科学家小组利用果蝇(Drosophila melanogaster)研究巨噬细胞如何激活,从而响应损伤或感染。据悉,利用果蝇,研究人员能够获得免疫细胞在活体中迁移时的动态行为的延时影片;同时,还能够轻松操纵不同的基因和信号通路,从而检测哪些基因对免疫细胞的行为重要。
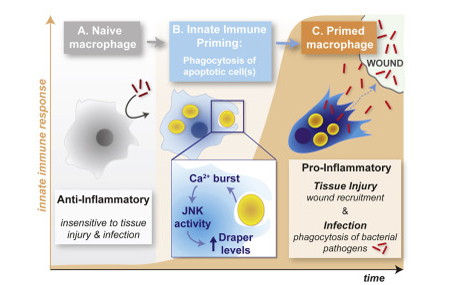
研究中,科学家们剖析了免疫细胞产生的分子记忆,结果发现,吞噬垂死细胞能够通过钙离子增加激活JNK信号,从而导致巨噬细胞中重要损伤受体Draper的数量增加。高水平的Draper能够使准备中的巨噬细胞感知到损伤信号。如果没有这一启动过程,免疫细胞无法“看见”创伤和感染。
论文的共同通讯作者Will Wood教授表示,利用果蝇研究人类疾病似乎是一种相当奇怪的方法,但这是一项理解免疫细胞行为方面激动人心的进展,也将帮助我们设计影响免疫细胞行为的新疗法。
作者: ipsvirus 时间: 2016-6-13 16:51
Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response
Helen Weavers, Iwan R. Evans, Paul Martin, Will Wood
Highlights
•Phagocytosis of apoptotic cells primes macrophages for future inflammatory response
•Naive macrophages are insensitive to tissue damage and bacterial infection
•Corpse uptake triggers macrophage calcium bursts that potentiate priming
•Calcium-induced JNK primes macrophages by upregulating the damage receptor Draper
Summary
Macrophages are multifunctional cells that perform diverse roles in health and disease. Emerging evidence has suggested that these innate immune cells might also be capable of developing immunological memory, a trait previously associated with the adaptive system alone. While recent studies have focused on the dramatic macrophage reprogramming that follows infection and protects against secondary microbial attack, can macrophages also develop memory in response to other cues? Here, we show that apoptotic corpse engulfment by Drosophila macrophages is an essential primer for their inflammatory response to tissue damage and infection in vivo. Priming is triggered via calcium-induced JNK signaling, which leads to upregulation of the damage receptor Draper, thus providing a molecular memory that allows the cell to rapidly respond to subsequent injury or infection. This remarkable plasticity and capacity for memory places macrophages as key therapeutic targets for treatment of inflammatory disorders.
http://www.cell.com/cell/abstract/S0092-8674(16)30494-9?_returnURL=http%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867416304949%3Fshowall%3Dtrue
| 欢迎光临 中国病毒学论坛|我们一直在坚持! (http://bbs.virology.com.cn/) |
Powered by Discuz! X3.2 |